Transcranial magnetic stimulation (TMS) has already changed the landscape for people with depression who do not respond to medications or traditional talk therapy. Accelerated theta-burst stimulation (aTBS) is now pushing that progress even further, showing that it may be possible to deliver stronger antidepressant effects in a shorter amount of time—using technology that looks a lot like the TMS we already use in clinic.
What Is TMS and Why Does It Matter?
TMS is a noninvasive neuromodulation treatment that uses pulsed magnetic fields to stimulate specific regions of the brain involved in mood regulation, typically the left dorsolateral prefrontal cortex (DLPFC).
Key points about standard TMS:
- It does not require anesthesia or surgery.
- Patients are awake and can return to normal activities after treatment.
- A typical FDA‑cleared course for depression is 5 sessions per week for 6–8 weeks (often 30–36 sessions total).
For many people with major depressive disorder who have not responded to multiple medications, TMS offers a chance at meaningful symptom relief without systemic side effects. But the time commitment can be intense—multiple weeks of daily visits—and some patients remain partially or fully treatment‑resistant.
What Is Theta-Burst Stimulation (TBS)?
Theta-burst stimulation is a specific pattern of TMS pulses designed to mimic the brain’s natural rhythms. Instead of delivering a continuous train of pulses over several minutes, theta-burst uses very short bursts:
- Bursts of 3 pulses at 50 Hz
- Repeated 5 times per second (at 5 Hz, in the theta range)
Intermittent theta-burst stimulation (iTBS) applied to left DLPFC has been shown to produce antidepressant effects comparable to standard 10 Hz TMS—but in a fraction of the time per session.
In clinical practice this means:
- Individual sessions may take only a few minutes.
- The shorter session length opens the door to multiple treatments per day, which is where accelerated protocols come in.
The Accelerated TBS Trial: Design and Real-World Relevance
A recent randomized, triple‑blinded, sham‑controlled trial evaluated a pragmatic accelerated theta-burst stimulation (aTBS) protocol in adults with treatment‑resistant depression (TRD).
Key design features:
- Population: 100 adults with TRD (multiple prior treatment failures).
- Protocol: 45 total sessions delivered as
- 3 iTBS sessions per day
- Over 15 consecutive weekdays (approximately 3 weeks).
- Target: Left dorsolateral prefrontal cortex (DLPFC), using standard scalp-based targeting rather than neuronavigation.
- Masking: Triple‑blinded—patients, treating clinicians, and raters were blinded to condition.
- Comparison: Active aTBS vs sham stimulation.
The “pragmatic” aspect is important. This was not a highly exotic, lab‑only protocol requiring special equipment or sophisticated imaging; the parameters were chosen to be feasible in real‑world TMS centers.
Defensible Outcomes: How Much Did Symptoms Improve?
The primary outcome was change in depression severity on the Hamilton Depression Rating Scale (HDRS‑17).
Results:
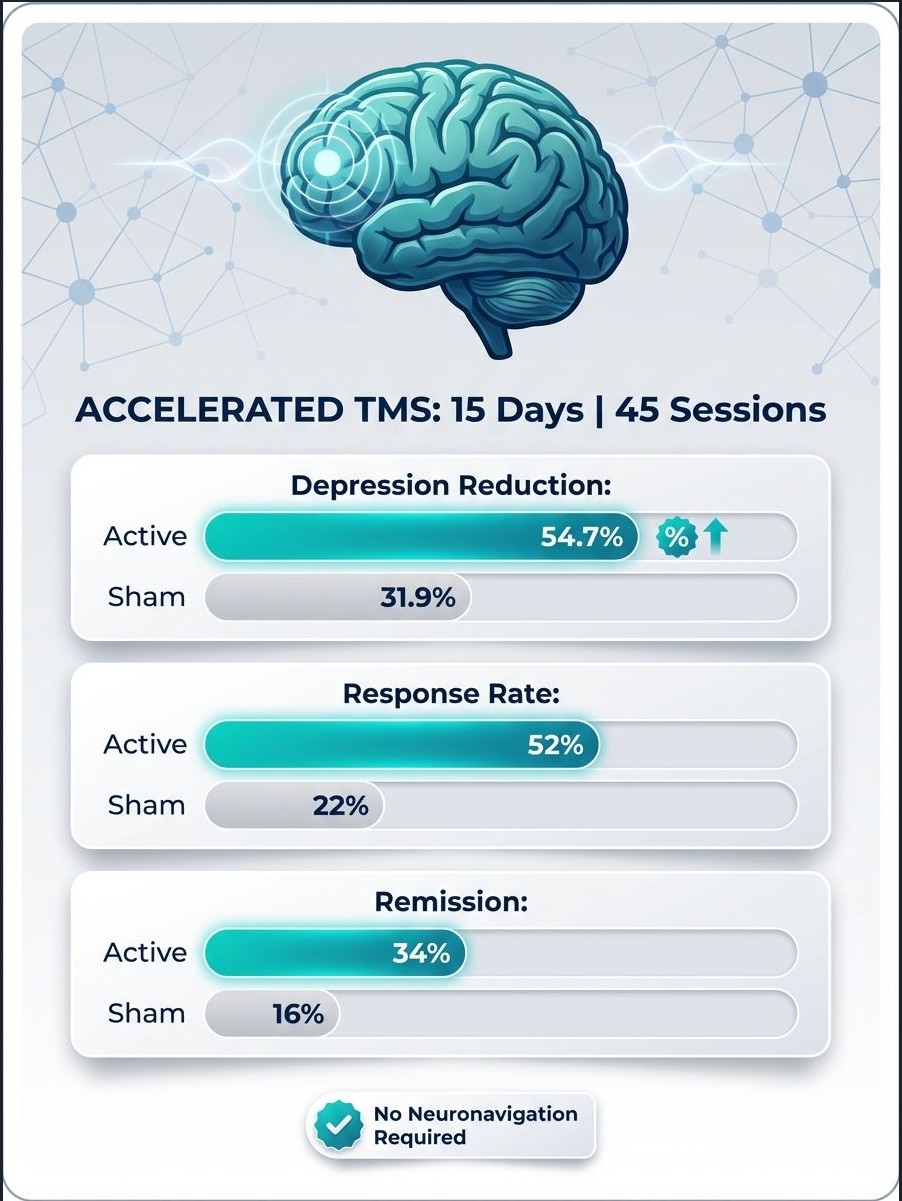
- HDRS‑17 reduction:
- Active aTBS: 54.7% average reduction in depressive symptoms
- Sham: 31.87% reduction
- Effect size: Cohen’s d = 0.65 (a medium-to-large effect)
Clinically important outcomes:
- Response (≥50% reduction in HDRS‑17):
- Remission (HDRS‑17 < 8):
In other words, more than half of patients receiving accelerated TBS achieved at least a 50% reduction in depressive symptoms, and roughly one in three reached remission—even though all had treatment‑resistant depression at baseline.
Safety and Tolerability
Safety is a central question for any intensified neuromodulation protocol. In this trial, accelerated TBS was well tolerated:
- The most common side effect was mild scalp pain, reported in 17.4% of patients in the active group versus 4.4% in the sham group.
- No seizures were reported.
- No manic episodes or serious adverse events were attributed to the treatment.
This aligns with the broader safety profile of TMS, which has a very low risk of serious events when delivered within established guidelines.
What Happens When Sham Non-Responders Get Real aTBS?
An important part of the study looked at patients who did not respond in the sham arm. They were then offered open‑label active aTBS:
- Approximately 75% of these sham non-responders experienced significant improvement once they received actual accelerated theta-burst stimulation.
This suggests that the protocol has meaningful therapeutic potential even in patients who initially “failed” the sham condition and underscores that the effect is not just placebo.
Why Accelerated TBS Matters for Clinical Practice
From a clinical and operational standpoint, accelerated TBS directly addresses several real‑world barriers in treatment‑resistant depression care:
- Time efficiency
- Traditional TMS protocols require 6–8 weeks of daily sessions.
- Accelerated TBS compresses a full course into about 3 weeks by stacking multiple short sessions per day.
- Scalability
- The protocol uses standard TMS hardware and scalp‑based targeting, so it can be deployed in existing TMS centers without neuronavigation.
- Patient adherence
- Shorter overall treatment timelines can improve adherence, especially for working professionals, caregivers, or patients traveling from out of town.
- Potential synergy with other interventions
- Intensified neuromodulation can be combined with psychotherapy, medication optimization, or even emerging interventions aimed at neuroinflammation and neuroplasticity, aligning with integrative brain health models.
How This Connects to Mind Spa Denver’s Approach
At Mind Spa Denver, the focus is on stacking evidence‑based, brain‑directed treatments to improve outcomes for people with depression, anxiety, PTSD, and traumatic brain injury. TMS is a core part of that strategy because it:
- Targets the circuits involved in mood and cognitive control.
- Avoids systemic side effects associated with many medications.
- Can be combined with ketamine, psychotherapy, and other neuroplasticity‑enhancing interventions in a structured program.
Accelerated theta-burst stimulation builds on this foundation by suggesting that:
- Higher “dose density” over a shorter period may produce faster and stronger antidepressant responses in TRD.
- Real‑world clinics can adopt these protocols with existing equipment and training, pending guideline updates and payer recognition.
For patients, the message is hopeful but measured: accelerated TBS is not a cure‑all, and not every patient will respond. Yet for people who have already tried multiple medications and standard therapies, this study shows that their brain is still plastic and responsive to targeted stimulation when delivered in an optimized schedule.
Key Takeaways for Patients and Clinicians
- Accelerated theta-burst TMS is a next‑generation, evidence‑based option for treatment‑resistant depression.
- The protocol tested (3 sessions/day for 15 days, 45 total) produced:
- ~55% mean symptom reduction,
- 52% response rate,
- 34% remission rate,
- With good tolerability and no serious adverse events in the trial.
- It fits into a broader shift in psychiatry toward faster, circuit‑targeted, and biologically informed interventions, rather than relying solely on incremental medication changes.
For individuals and families exploring options beyond traditional antidepressants, accelerated TMS and related neuromodulation strategies represent a promising new frontier—one that aligns with Mind Spa Denver’s mission to combine advanced technology, neuroscience, and compassionate care to help patients get better, faster.
Works Cited
- Ramos MRF, Goerigk S, da Silva VA, et al. Accelerated theta‑burst stimulation for treatment‑resistant depression: randomized clinical trial. JAMA Psychiatry. 2025.
- Home‑based and clinic‑based TMS/tDCS neuromodulation over left DLPFC for major depression. Nature Medicine and related neuromodulation literature.
- Neuroinflammation and stress‑induced pathophysiology in major depressive disorder: mechanisms and therapeutic implications. Frontiers in Cellular Neuroscience. 2025.
- Should inflammation be a specifier for major depression in the DSM? JAMA Psychiatry. 2025.
- Pragmatic accelerated theta-burst stimulation shows promise for treatment‑resistant depression. UTHealth Houston Psychiatry news release. 2025.